IG TECHNOLOGY LTD
Overcoming through innovation
Features X
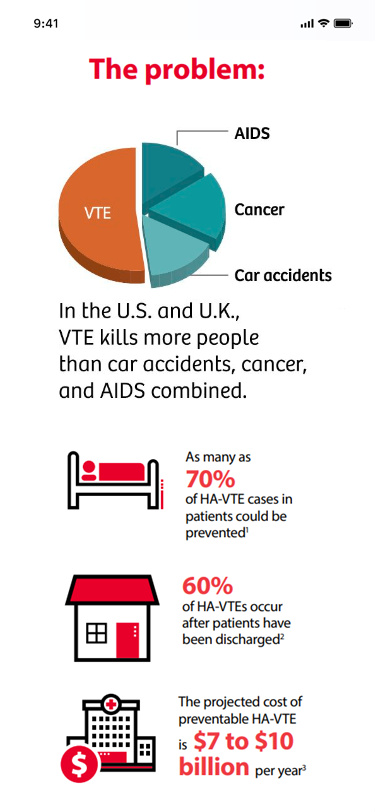
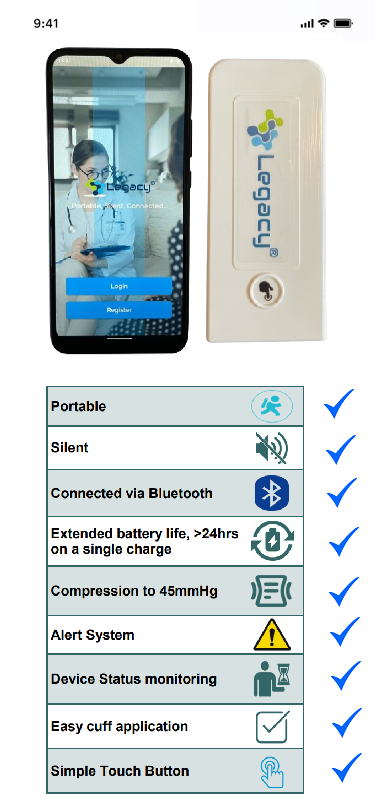


The VTE problem
Every 37 seconds someone in the western world dies from a venous thromboembolism (VTE).
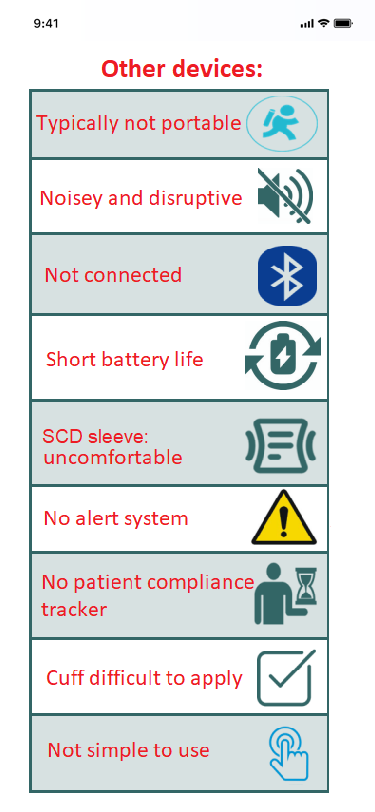
The challenges
Overcoming VTE is not without its challenges. Legacy wins through innovation where others fail.
Legacy the solution
Introducing Legacy IPC, a ground-breaking solution revolutionising the prevention of VTE.
Lightweight and portable
Legacy IPC is lightweight, compact and portable. It weighs just 70g!
Completely silent operation
Experience the unparalleled comfort of Legacy IPC's completely silent treatment.
Connected!
With integrated Bluetooth connectivity, Legacy IPC enables seamless monitoring and control.
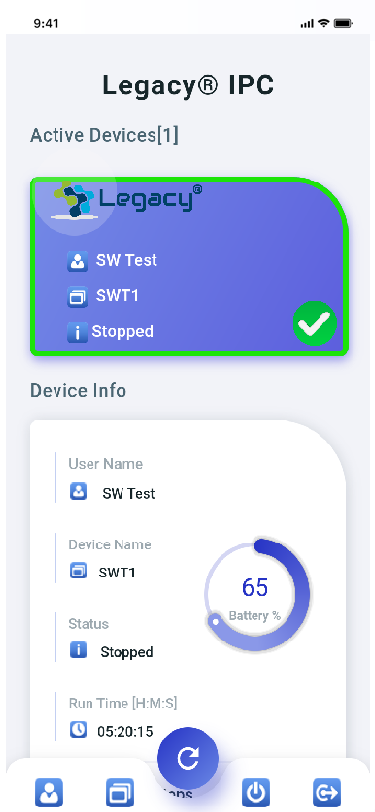
LEGACY® MOBILE APP
The Legacy® IPC mobile application offers a range of benefits for users of the intermittent pneumatic compression device. By providing a user-friendly interface, the app simplifies monitoring and tracking of compression therapy sessions. Users can conveniently access real-time data, including session history and progress tracking, allowing for better management and adherence to the treatment plan. Additionally, the app offers helpful reminders and notifications, ensuring that users stay on track with their therapy. With the Legacy IPC mobile app, monitoring and optimizing the effectiveness of your treatment has never been easier!
[User feedback was considered during APP development]
Legacy® IPC kit

The Legacy® IPC kit contains the following items:
- Legacy® IPC Pump x 2
- Legacy® Bladder (LEFT/RIGHT)
- Legacy® Comfort Sleeve (LEFT/RIGHT)
- Power Supply x 1
- Dual USB Power Lead x 1
- Legacy® Instructions for Use x 1
The Legacy® Portable IPC
Legacy® is a medically approved Intermittent Pneumatic Compression (IPC) device used to help prevent prevent blood clots in the deep veins of the legs. The device uses cuffs that fill with air to squeeze the soleus and gastrocnemius muscles, simulating the effects of walking. This increases blood flow through the veins of the legs and helps to prevent the formation of blood clots and stasis.
DistributorsIntended users
Legacy® is to be used as an aid in the prophylaxis for DVT by persons expecting to be stationary for long periods of time during hospital admission or home recovery.
Indications for use
The Legacy® IPC is a prescription device intended for the prophylaxis of Deep Vein Thrombosis (DVT), stimulating venous and arterial circulation, aiding in the prevention of venous stasis ulcers, aiding in the healing of cutaneous ulcers, reducing acute/chronic edema and compartmental pressures. For use in home or hospital settings.
Contraindications
Use of this device is contraindicated for patients with any of the following conditions:
- Infections in the limb, including cellulitis, without appropriate antibiotic coverage.
- The presence of lymphangiosarcoma.
- Suspicion or confirmation of the presence of Deep Vein Thrombosis (DVT).
- Inflammatory phlebitis or episodes of pulmonary embolism.
- Congestive Heart Failure (CHF).
- Pulmonary edema.
- Severe arteriosclerosis or other ischemic vascular disease.
- Any local condition of the extremity that would interfere with its application, including but not limited to : dermatitis, immediately following vein ligature, gangrene, skin grafts, casts or splints, open wounds.
THE LEGACY® IPC
Legacy® is without a doubt a revolution in IPC treatment. The freedom that it offers patients is next to none.

Sandra Forde
Business Development ManagerLegacy® is amazing! it is easy to apply and completely silent whilst running. A real game changer.

Kari Golgan
Deputy Sister Charge NurseThe Legacy® device offers unrivalled comfort. The APP is also extremely useful and allows us to track patient compliance with ease.

Abbey Goacher
Registered Nursing AssociateLEADERSHIP TEAM

Mr Ivan Green
Chief Executive Officer

Dr Stephen Ingram
Chief Technical Officer

Mr Eliot Clare
Chief Commercial Officer
ABOUT US
Who are we?
IG-Technology Ltd is a medical product development and manufacturing company dedicated to merging state-of-the-art technology with technical expertise. Our overarching mission is to enhance the well-being of individuals by delivering innovative solutions that significantly advance healthcare.
The Legacy® IPC
Introducing the Legacy® IPC, a medically approved Intermittent Pneumatic Compression (IPC) device specifically tailored to tackle the prevalent issue of poor patient compliance in both the clinical and health homecare settings. With a focus to improve patient comfort and continuous care, the Legacy® IPC provides the most compact and lightweight wearable IPC on the market. The use of novel pump technology allows for absolute silent operation, empowering patients to maintain their mobility without any disturbance both day and night. It’s not just the health of the patient we consider but also the environment by minimizing plastic waste attributed to disposable items.
Harnessing the power of Bluetooth connectivity, our solution enables monitoring of multiple devices status and compliance. Data is effortlessly transferred in real time, empowering clinicians to promptly identify and address any abnormalities, thereby optimizing patient health outcomes.